Introduction
According to the current study, stimulating a particular brain wave with light and sound treatment improves the release of peptides from interneurons, facilitating the elimination of proteins linked to Alzheimer’s disease via the brain’s lymphatic system.
Studies conducted at MIT and other universities are beginning to demonstrate that sounds and light flashing at the 40 Hz gamma brain rhythm frequency can both reduce the symptoms of Alzheimer’s disease (AD) in laboratory mice and human volunteers, as well as decrease the illness’s development.
Researchers at the Picower Institute for Learning and Memory at MIT have uncovered a crucial mechanism that may be responsible for these positive effects in a new study published in Nature. This mechanism involves the brain’s glymphatic system, a recently discovered “plumbing” network that runs parallel to the blood vessels in the brain, clearing amyloid proteins, a hallmark of AD pathology.
“Many have asked me how it works ever since we released our initial findings in 2016. Why is 40 Hz used? Why not use a different frequency? MIT’s Ageing Brain Initiative head, Li-Huei Tsai, a Picower Professor of Neuroscience and senior author of the study, stated. “We have worked very diligently in the lab to address these extremely significant questions,” the scientist said.
The new paper reports a series of experiments conducted under the direction of Mitch Murdock, an MIT doctoral student studying brain and cognitive sciences, that demonstrate that a specific type of neuron releases peptides in response to an increase in 40 Hz power and synchrony in the brains of mice stimulated by sensory gamma stimulation. The study’s findings also imply that such brief protein signals subsequently trigger certain actions that encourage the glymphatic system to facilitate increased amyloid clearance.
Murdock, who was co-supervised by Tsai and co-author and collaborator Ed Boyden, Y. Eva Tan Professor of Neurotechnology at MIT, a member of the McGovern Institute for Brain Research, and an affiliate member of The Picower Institute, stated, “Yet, our tests’ results validate this elimination mechanism via the principal glymphatic channels.
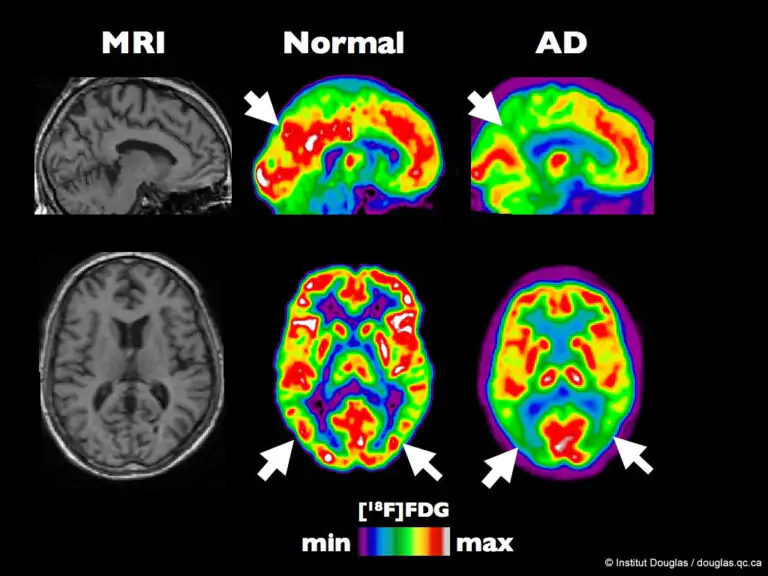
From Gamma to Glymphatics
The glymphatic system is a major pathway for the clearance of brain waste and may be regulated by brain rhythms, so Tsai and Murdock’s team postulated that this could help explain the lab’s earlier findings that gamma sensory stimulation lowers amyloid levels in Alzheimer’s model mice.
Murdock and co-authors first reproduced the lab’s previous findings that 40 Hz sensory stimulation enhances 40 Hz neuronal activity in the brain and lowers amyloid levels in “5XFAD” mice, which is genetically the same as Alzheimer’s disease. The next step was to see whether the fluids that pass through the glymphatic system to remove waste had changed in any way that might be associated with. In fact, they found that, in comparison to untreated controls, mice given sensory gamma stimulation had higher levels of cerebrospinal fluid in their brain tissue. The pace at which interstitial fluid is evaporating from the brain was also seen to be increasing. Additionally, he observed larger lymphatic channel diameters in the gamma-treated mice and higher amyloid buildup in the cervical lymph nodes, which serve as the drainage location for that flow.
The scientists concentrated on the aquaporin 4 (AQP4) water channel of astrocyte cells, which allows the cells to enhance glymphatic fluid exchange, in order to determine how this increased fluid flow may be occurring. By chemically inhibiting APQ4 activity, scientists stopped sensory gamma stimulation from declining amyloid levels and from improving learning and memory in mice. And then, as an extra test, they interfered with gamma-driven amyloid clearance by using a genetic method to impair AQP4.
Further away from the promotion of fluid exchange by APQ4 activity in astrocytes, gamma waves also increase the pulsing of nearby blood vessels, which is another way in which they facilitate glymphatic flow. In comparison to untreated controls, mice exposed to sensory gamma stimulation exhibited more arterial pulsatility in a number of tests.
One of the most effective novel methods for monitoring the effects of a stimulus, such as sensory gamma stimulation, on various cell types is to sequence their RNA and monitor changes in gene expression patterns. Tsai and Murdock’s mates observed using this technique that gamma sensory stimulation did, in fact, cause changes compatible with elevated astrocyte AQP4 activity.
In response to peptides
The RNA sequencing data also showed that a subpopulation of neurons known as “interneurons” produced a noticeable increase in several peptides in response to gamma sensory input. Although peptide release is known to be reliant on brain rhythm frequencies, this was nonetheless noteworthy since VIP, one specific peptide, is linked to advantages in the battle against Alzheimer’s and aids in the regulation of vascular cells, blood flow, and lymphatic clearance.
Using this intriguing discovery, the researchers carried out tests that revealed the brains of mice given gamma treatment had increased VIP. Using a peptide release sensor as well, the researchers discovered that sensory gamma stimulation increased the quantity of peptide released by VIP-expressing interneurons.
But did this release of gamma-stimulated peptides aid in the glymphatic clearance of the amyloid? To find out, the researchers carried out a second experiment in which they chemically switched off the VIP neurons. They found that when the mice were exposed to sensory gamma stimulation, they no longer exhibited an increase in arterial pulsatility or gamma-stimulated amyloid clearance.
According to Murdock, “we believe that many neuropeptides are involved.” Tsai continued, “Identifying what additional peptides or other molecular factors may be driven by sensory gamma stimulation will be a major new direction for the lab’s research.”
Tsai and Murdock noted that although the mechanism by which sensory gamma stimulation benefits the brain—the lymphatic clearance of amyloid—is the focus of this work, it’s probably not the only one. While the clearing effects shown in this study happened rather quickly, weeks or months of continuous sensory gamma stimulation have been required in lab trials and clinical investigations to have long-lasting impacts on cognition.
However, research into the potential benefits of sensory stimulation of brain rhythms for the treatment of neurological illnesses continues with each new study.
Conclusion:
The groundbreaking research conducted at MIT’s Picower Institute for Learning and Memory sheds light on the potential of sensory gamma stimulation at 40 Hz frequency to mitigate Alzheimer’s disease. Through cautious testing, the scientists uncovered a method by which the brain’s glymphatic system helps eradicate amyloid particles linked to the pathology of Alzheimer’s disease. Through the use of light and sound therapy to stimulate particular brain waves, the release of peptides from interneurons is improved, which facilitates the lymphatic system’s removal of toxic proteins. These studies uncover the importance of further research into the possible benefits of sensory stimulation on brain function and offer encouraging insights into novel treatment methods for Alzheimer’s disease.
FAQ's
Q: How does sensory gamma stimulation work in combating Alzheimer’s disease?
A: The brain is subjected to 40 Hz pulsing light and sound waves during sensory gamma stimulation. This stimulation causes interneurons to produce peptides, which in turn helps the brain’s lymphatic system get rid of amyloid proteins linked to Alzheimer’s disease.
Q: Why is 40 Hz frequency used for sensory gamma stimulation?
A: Scientists have found that 40 Hz brain stimulation improves neuronal activity and facilitates amyloid protein removal. It seems that this frequency works very well to produce the intended therapeutic outcomes.
Q: What role does the glymphatic system play in Alzheimer’s treatment?
A: The lymphatic system of the brain functions like a “plumbing” network, taking away harmful material like amyloid proteins. By improving glymphatic fluid flow, sensory gamma stimulation aids in the brain’s detoxification of toxic chemicals.
Q: How do these findings translate to potential treatments for Alzheimer’s patients?
A: The recent discovery of the process linking amyloid clearance with sensory gamma stimulation provides a positive procedure for the establishment of new Alzheimer’s disease treatments. For a more thorough study of the long-term impacts and therapeutic possibilities of this strategy in medical settings, more investigation is required.







This webpage is outstanding. The site owner’s passion is evident in the excellent content. I’m in awe and anticipate reading more amazing pieces like this one.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Dating is a truly joyful experience. Sometimes we lose sight of this truth in our search for the right Online dating site
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.